Battling the Brain Bleed: A Neuro-ICU Success Story
Introduction
In the high-stakes world of intensive care, seconds matter and decisions define outcomes.
Today’s story takes us inside the ICU, where a 51-year-old woman fought one of the deadliest brain emergencies: a massive hypertensive intracerebral hemorrhage with intraventricular extension.
This is more than just a medical case — it’s a tale of swift action, teamwork, and critical thinking.
Patient Profile
Age/Sex: 51-year-old female
Known History: Long-standing uncontrolled hypertension
Presentation: Sudden onset headache, vomiting, right-sided weakness, and altered consciousness for 2 hours before hospital arrival.
Initial Vitals: BP 210/120 mmHg, HR 96 bpm, RR 18/min, SpO₂ 97% RA, Temp 98.6°F.
GCS on arrival: E2V2M4 (Total 8/15)
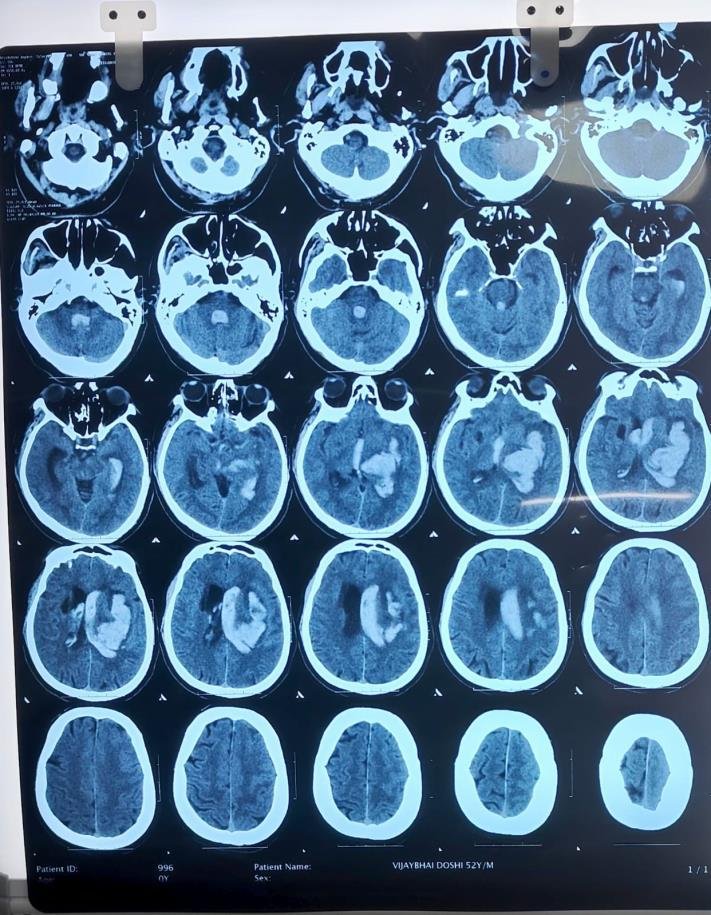
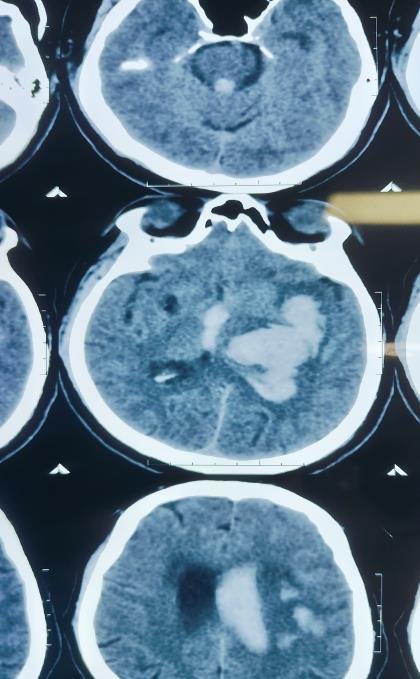
CT Scan Findings
Acute large intraparenchymal bleed in the left gangliocapsular region, corona radiata, and left fronto-parietal lobe.
Size: 41 × 57 × 60 mm (CC × AP × TR).
Mild perilesional edema causing mass effect with 6.7 mm midline shift to the right.
Intraventricular extension of bleed into both lateral ventricles, third, and fourth ventricles.
Mild periventricular ooze → suggestion of developing hydrocephalus.
Mild cerebral edema.
1. Summary
This was a case of hypertensive intracerebral hemorrhage (ICH) with intraventricular extension, managed medically due to the patient’s deep location bleed and high surgical risk. The ICU goal was ABC stabilization, ICP control, BP optimization, prevention of secondary brain injury, and complication management.
2. Definition
Intracerebral Hemorrhage (ICH): Bleeding within the brain parenchyma.
Intraventricular Hemorrhage (IVH): Blood extends into the brain’s ventricular system.
Hypertensive ICH: Bleed due to chronic hypertension-induced small vessel rupture, often in deep brain structures (basal ganglia, thalamus, pons, cerebellum).
3. Etiology
Primary cause here: Chronic uncontrolled hypertension → Charcot-Bouchard micro aneurysm rupture.
Other causes (not in this patient): Amyloid angiopathy, AV malformation, anticoagulant therapy, trauma, tumor bleed
4. Pathophysiology (Specific to This Case)
- Chronic Hypertension → Vessel Wall Weakness: Small penetrating arteries in
basal ganglia undergo hyaline arteriosclerosis. - Sudden BP Surge: Vessel rupture → rapid hematoma expansion in deep
parenchyma. - Mass Effect: Surrounding brain compression, midline shift, raised ICP.
- Ventricular Extension: Blood blocks CSF flow → risk of acute hydrocephalus.
- Neuroinflammatory Cascade: Hemoglobin breakdown products trigger edema and
secondary neuronal injury.
5. Clinical Features
Symptoms: Sudden headache, vomiting, hemiparesis, altered sensorium.
Signs: Right-sided weakness, facial deviation, GCS drop, high BP, no signs of trauma.
6. Diagnostics
Neuroimaging: Non-contrast CT brain → gold standard for acute bleed.
Baseline Labs: CBC, LFT, RFT, coagulation profile, electrolytes, ABG, Troponin I, Blood group.
ICP Monitoring: Clinical + imaging-based (no invasive ICP monitor here).
7. Treatment – Day-by-Day ICU Journey
- Day 1 – Admission & Stabilization
- Airway:
- Patient presented with irregular, gasping breathing pattern and significant risk of aspiration.
- Intubated with ET tube size 7.5 mm, fixed at 22 cm at the lip for airway protection and to facilitate controlled ventilation.
- Aspiration of gastric contents noted during intubation → immediate suctioning performed.
- Ventilation: Initiated on volume-controlled ventilation (FiO₂ 50%).
- Circulation:
- BP control with IV labetalol infusion targeting SBP 140–160 mmHg.
- Neuroprotection: Head end elevation 30°, normothermia maintained, sedation with midazolam.
- Lines & Monitoring: Central venous catheter inserted, Foley catheter placed for urine output monitoring, invasive arterial line for BP monitoring.
- Investigations:
- CBC, LFT, RFT, electrolytes, coagulation profile, Trop I, ABG, blood grouping.
- Nutrition:
- Ryle’s tube placed for enteral feeding initiation.
- Family Counseling:
- Detailed explanation of poor prognosis and ICU plan.
- Medication:
- Inj. Mannitol + glycerol IV QDS for ICP control
- Inj. Levetiracetam 1 g IV stat, then 500 mg IV TDS for seizure prophylaxis
- Inj. Monocef 2 g IV BD (empirical antibiotic)
- Inj. Pantoprazole 40 mg IV BD (stress ulcer prophylaxis)
- Inj. Emset 4 mg IV BD (antiemetic)
- Inj. Lasix 10 mg IV BD (diuresis and ICP management)
- IVF @ 50ml/HR
- Airway:
- Day 2 – Multimodal Support
- Neurological Status: No further deterioration, GCS stable at E4VTM6.
- DVT Prophylaxis: Anti-embolism stockings applied (no pharmacological prophylaxis due to ICH).
- Pulmonary Care: Regular endotracheal suction
- Nebulization: Duolin (ipratropium + salbutamol) TDS, Budecort (budesonide) BD
- Physiotherapy: Passive limb physiotherapy initiated.
- Skin Care: Air mattress to prevent bedsores.
- Nutrition: Ryle’s feeding 150 mL every 2 hours (isocaloric formula).
- Lab Follow-up: Repeat ABG, electrolytes correction as required.
- Day 3 – Bowel Management & Ongoing Care
- Same treatment continued with bowel care (Syp Cremaffin 20 mL HS).
- Neurological status: Gradual improvement in GCS, following commands, spontaneous eye opening.
- Weaning Trial: Patient passed spontaneous breathing trial with stable hemodynamics, good cough reflex, and adequate tidal volumes.
- Extubation Event – Evening, Day 3
- Decision Criteria Met:
- GCS improved, following commands
- SpO₂ >96% on minimal support
- Effective cough and secretion clearance
- Hemodynamically stable
- Procedure: Extubated to supplemental oxygen via face mask
- Outcome: No desaturation, no stridor, smooth transition to spontaneous breathing.
- Post-extubation Care: Nebulization, airway clearance physiotherapy, close monitoring for 24 hours.
- Same neurological care continued.
8. Complications to Watch
Acute hydrocephalus, Rebleed, Herniation syndromes, Aspiration pneumonia, DVT & pulmonary embolism, Seizures
9. Study Triad for Hypertensive ICH
“Headache – Vomiting – Focal Neuro Deficit”
Mnemonic: HVF = “Hemorrhage Very Fast”
10. Fun Fact
Deep ICHs in basal ganglia are twice as common in hypertensive patients compared to normotensives — and often present in the early morning hours when BP surges are common.
11. Case Table – Pertinent Positives & Negatives
| Pertinent Positive | Pertinent Negative |
| Sudden neuro deficit | No trauma history |
| CT shows large basal ganglia bleed | No cortical infarct |
| Hypertension history | No anticoagulant use |
| Intraventricular extension | No SAH pattern |
12. Short Case Story – for Memory
“At 2 AM, Mrs. P, a 51-year-old, collapsed while preparing tea. Her husband rushed her in — half her body limp, eyes half-open, speech slurred. The CT scan lit up with a deep red bloom in her brain — a hypertensive time bomb that had just gone off. There was no time for surgery; the fight would be won with precision, patience, and protocol. For three days, the ICU became her shield, every drip, every monitor, every elevated headrest part of the battle plan. Slowly, the swelling eased, the bleed settled, and the warrior within her brain quieted down.”
13. Quick Q&A
- Qus: Why was craniotomy not done?
Ans: Deep basal ganglia bleeds carry high surgical morbidity; medical management
preferred unless there’s mass effect with rapid deterioration. - Qus: Why mannitol over hypertonic saline?
Ans: Mannitol acts faster for acute ICP reduction in this setting and patient’s sodium was within normal range. - Qus: Why give Cremaffin?
Ans: Prevents constipation → avoids Valsalva → prevents ICP spikes
15. References
AHA/ASA Guidelines for Management of Spontaneous ICH (2022)
UpToDate – Spontaneous intracerebral hemorrhage: Pathogenesis, clinical features, and diagnosis
WHO – Global Burden of Stroke Report
AMBOSS – Intracerebral Hemorrhage